Medizin muss heute innovationsgetriebener und personalisierter sein als je zuvor. Deshalb spielt Mikrofluidik bei der Erforschung neuer therapeutischer Ansätze eine entscheidende Rolle. CETONI hat eine klare Mission: Mit innovativen Lösungen befähigen wir unsere Kunden, nicht nur Teil der Entwicklung zu sein, sondern sogar den Takt vorzugeben.
Im Gegensatz zu herkömmlichen Methoden steht bei der individualisierten Medizin weniger die Krankheit, als vielmehr der Mensch im Mittelpunkt. Das vorrangige Ziel sind patientenorientierte Interventionen ohne Zeitverlust und Nebenwirkungen. Durch die Etablierung von In-vitro-Assays, die ein erster Schritt hin zu belastbaren Wirkungsprüfungen darstellen, ist nun der Weg bereitet, um personalisierte Therapien zielgerichtet weiterzuentwickeln und auf Hochdurchsatz hin zu optimieren
Herkömmliche Verfahren stoßen an ihre Grenzen
Zellkultivierungen werden üblicherweise in standartisierten Zellkulturflaschen in einer geregelten CO2-Umgebung durchgeführt. Dabei sorgt die geschlossene Inkubationseinheit für eine konstante Temperatur, hohe Luftfeuchtigkeit und Sterilität. Ein Problem stellt aber die räumliche Trennung der Zellkultur- und Auswerteeinheit dar, aufgrund derer keine ganzheitliche Aussage zum zellphysiologischen Prozess in Echtzeit getroffen werden kann. Mikrofluidische Systeme hingegen eignen sich hervorragend zur gleichzeitigen Analyse verschiedener Testsubstanzen. Daher rückte die Entwicklung einer automatisierten Zellkultur zur Reduktion von Kosten und zeitintensiven Arbeiten sowie zur Echtzeitbeobachtung von Zellen, gerade in Hinblick auf die Entwicklung personalisierter Therapien, in den Mittelpunkt des Interesses.
Die Miniaturisierung eines Laborablaufes von standardisierten In-vitro-Assays im Well-Format auf einen Lab-on-a-Chip System (LoC) ist nach einer kurzen Implementierungsphase äußerst zeit- und kostensparend. Durch die reduzierten Analyseflächen im Chip ist ein geringerer Probeneinsatz notwendig. Diese neue technologische Möglichkeit liefert insbesondere hinsichtlich der Zellkultivierung neue Perspektiven. Dabei können solche Systeme nicht nur den Zelleinsaatprozess automatisiert realisieren und die Versorgung des Zellkulturmediums mit Nährstoffen sicherstellen, sondern auch die toxische Stimulation der Zellen in Echtzeit gewährleisten. Durch die Verwendung kleinster Zellkulturflächen wird nur eine geringe Anzahl an Zellen benötigt, die bei kontrollierter Zellversorgung (automatisiertes Pump-, Versorgungssystem und Heizeinheit) in Echtzeit (Real-Time) untersucht werden können. Das Pumpsystem erlaubt zudem eine automatisierte Kultivierung.
Die Standardkultivierung erfordert üblicherweise ca. 2-4 μl Zellkulturmedium pro 100-1000 Zellen/mm2, wohingegen bei mikrofluidischen Zellkulturen (abhängig vom Design des Systems) ca. nur 60nl Zellkulturmedium pro 200 Zellen/mm2 benötigt werden [1]. Besonders bei anspruchsvollen Kulturen mit teuren Kulturmedien bedeutet dies ein enormes Einsparpotenzial.
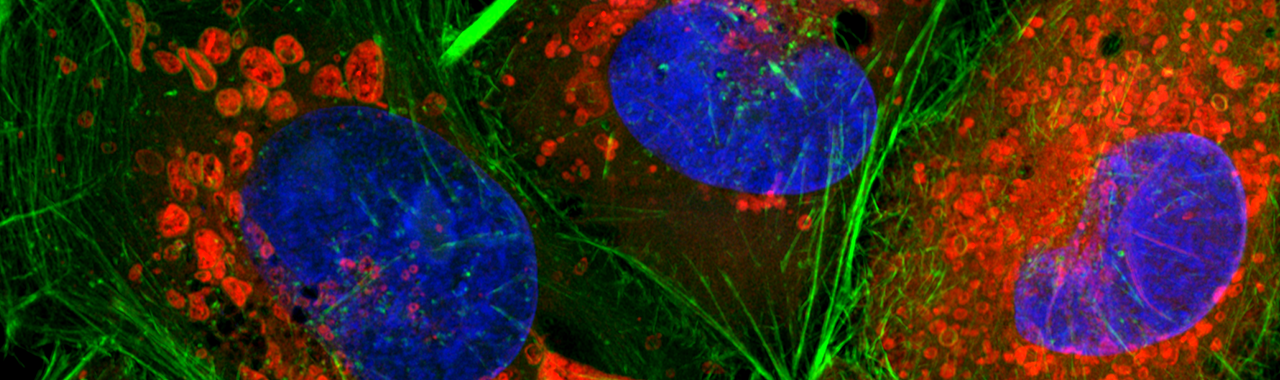
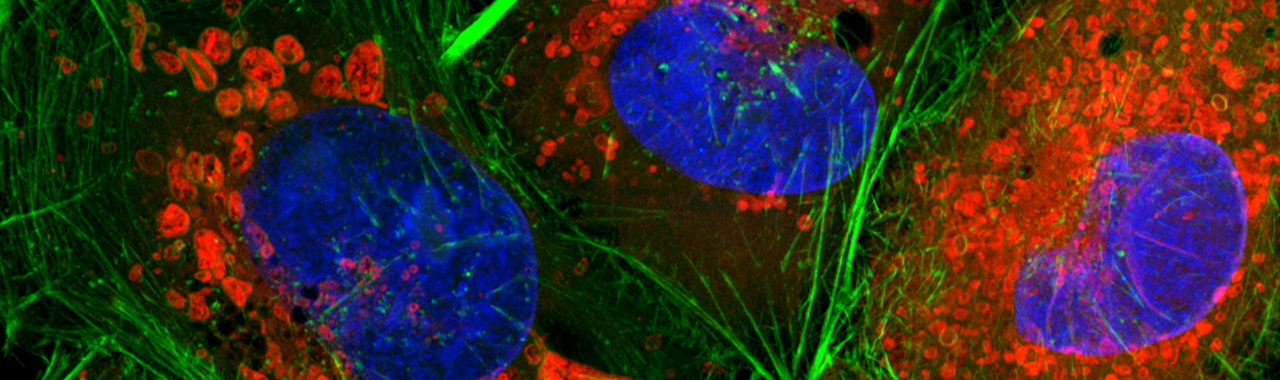
Fluoreszenzfärbung von HeLa-Zellen. Blau: Kerne (DAPI), Rot: Mitochondrien (Mitotracker), Grün: Aktin (Phalloidin)
Quelle: Astrid Pflieger (Ernst-Abbe-Hochschule Jena / Institut für Mikrosystem- und Präzisionsfertigungstechnik)
Das Potenzial der Skalierung mikrofluidischer Zellkultivierung ist enorm
Aufgrund der Parallelisierung der mikrofluidischen Kanalstrukturen ist ein Hochdurchsatz-Screening größerer Probenmengen bei gleichzeitiger Durchführung mehrerer unabhängiger Assays in Echtzeit möglich.
In den vergangenen Jahren wurde mit der Implementierung herkömmlicher in vitro Tests in LoC-Systeme die Ära der Biomikrosystemtechnik revolutioniert und grundlegend nachhaltig verändert. Dabei umfasst das Repertoire der etablierten Testsysteme simple mikrofluidische Zellmodelle zum Aufbau verschiedener Zellstrukturen (2D, 3D, Sphäroide), bis hin zu zellularen Perfusionsmodellen zur Mimikry biologischer Strukturen und letztendlich Testung verschiedener Substanzen am patientennahen Zellmodell. Durch die Etablierung einer anspruchsvollen Kultur zum Aufbau realistischer Gewebefunktionen zur in vitro Testung z.B. von Medikamenten können ganz einfach patientennahe Wirksamkeitstest durchgeführt werden.
 Die Zukunft der individualisierten Medizin, Pharmakologie oder Toxikologie
Die Zukunft der individualisierten Medizin, Pharmakologie oder Toxikologie
Ein LoC bildet folglich eine wertvolle Basis für die Entwicklung patientenbezogener Therapien für die individualisierte Medizin, Pharmakologie oder Toxikologie. Die Kombination von zweidimensionalen Zellkulturen und LoC-Systemen stoßen somit auch in der pharmazeutischen Industrie und medizinischen Forschung auf ein rasant wachsendes Interesse.
Dreh- und Angelpunkt zur Realisierung erfolgreicher Lab-on-a-Chip-Verfahren sind hochpräzise und pulsationsfreie Dosiersysteme, um u.a. die exakte Dosierung pharmakologischer Substanzen zur Wirksamkeitsüberprüfung zu gewährleisten und gleichzeitig Scherstress für die Zellen so gering wie möglich zu halten. Die äußerst kompakten Dosiersysteme von CETONI wurden speziell für diese Anforderungen entwickelt und ermöglichen dank des zukunftsweisenden Zusammenspiels von Hochpräzisionspumpen, Conti-Flow-Ventilen und intelligenter Software die Realisierung präzisester – kontinuierlicher – Fluidströme. Selbst die Anbindung unterschiedlichster Detektions- und Analysesysteme kann problemlos ermöglicht werden.
[1] S. Halldorsen, E. Lucumi, R. Gomez-Sjoberg and R. M. Fleming. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices, Biosens Bioelectron, vol. 63, pp. 218-31, 2015.