Cell-on-Chip: More and more research fields aim to miniaturize and transfer cell cultures and complete assays to a lab-on-a-chip system. On the one hand, the reduced analysis areas mean that less sample is required, and on the other hand, it is possible to observe cell behaviour in real time. High-precision and pulsation-free dosing systems are the key to successful implementation of such lab-on-a-chip procedures. Our CETONI nemesys syringe pumps are used in numerous laboratories for the realization of microfluidic analyses.
We have therefore compiled 10 tips from which beginners and experienced researchers can benefit in their work.
1. The right chip material
The demands on the chip material for cell cultivation are high. The material should not only be biocompatible, but also have a high transmission property. For this reason, most of the work is based on the polymers PDMS (polydimethylsiloxane) or COC (cycloolefin copolymers). Nevertheless, PDMS also has some disadvantages due to its gas permeability and it is not resistant to chemicals, which means that COC or glass are becoming more and more important.
2. CO2-independent cell culture media
CO2-independent media are ready-to-use formulations which independently build up HEPES-(2-(4-(2-hydroxyethyl)-1-piperazinyl)-ethanesulfonic acid)- a buffer system, e.g. based on mono- and di-basic sodium phosphate and β-glycerophosphate. Therefore, the supply of CO2 is not necessary to maintain the buffer system.
3. Separated cell reservoirs
The use of cavities (lowered cell reservoirs) can both create a microclimate within the cell reservoir and protect the cells from shear stress, both of which are otherwise disadvantages of flow cell cultivation.
4. Coating favours cell adhesion
Many different wet chemical coating substances are known to promote cell adhesion. Collagen, gelatine or the substance mixture Matrigel® are most commonly used. Depending on the cell culture used, the suitability must be investigated on the basis of a time-dependent growth profile.
5. Exact control of the flow
A too high flow rate can not only prevent the adhesion of the cells, but can even detach them, both of which are a consequence of the shear stress that is too high in this case. A flow rate that is too low, on the other hand, would not mean a sufficient supply of nutrients for the cells. Ultimately, a compromise must be chosen in which the flow rate is adapted to the glucose consumption of the cells.
6. Constant temperatures
Miniaturized cell-based sensors allow the holistic statement on cell physiological processes in real time through incubator-independent observation. However, this requires the maintenance of 37 °C for the cultivation of human cells. In this respect, different approaches are taken when heating an incubator-independent system. Peltier elements, heating foils or slides, which have been provided with an ITO (Indium Tin Oxide) coating. The ITO coating offers an even temperature distribution and an excellent optical transparency for simultaneous light microscopic examinations of the cell culture.
7. Eliminating gas bubbles
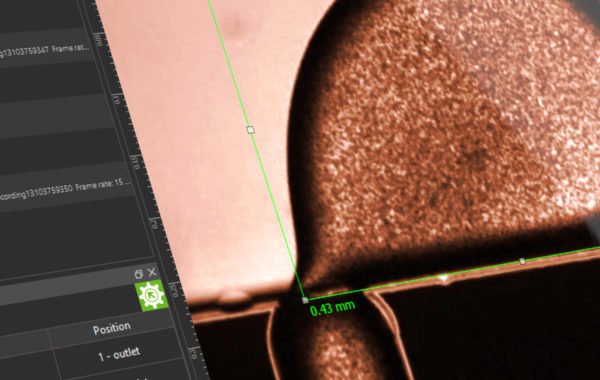
In addition to the general aim of air-free filling of the system, the greatest challenge in microfluidics is to reduce remaining gas bubbles in the chip system. Very often media are degassed beforehand or so-called “bubble traps” (degasser) are used in the process. These not only represent an expense in terms of equipment and a potential risk of contamination, but also make pulsation-free conveying more difficult. Another approach makes use of Henry’s law, where the proportion of gas dissolved in a liquid is proportional to the pressure. In a figurative sense, the increase in pressure in the system favours the solubility of the smallest gas bubbles in the system, which thus lose their disturbing effect. This effect can be exploited by the correct placement of a back-pressure regulator (BPR), which provides a fluidic resistance and thus ensures the desired pressure increase depending on the set flow rate.
8. Continuous-flow
In order to expose cells to conditions as homogeneous as possible and to supply them continuously with nutrients, a continuous and low-pulsating flow is essential. Subsequent incubation with appropriate test substances allows meaningful and reproducible results to be generated afterwards.
9. Reduce the risk of contamination
A contaminated cell culture is a nightmare for every cell researcher. Especially in incubator-independent cell-on-chip research, the risk of contamination is correspondingly high and should be monitored particularly carefully. The use of special sterile filters before and after the chip system supports contamination-free cultivation. Special attention should of course be paid to the fluid connection technology and the conveyor system. Through the targeted use of disposable or autoclavable components such as syringes and valves, the modular nemesys syringe pumps are ideally suited for this purpose.
10. The cell seeding
The cell seeding process in the chip system is particularly critical because the uniform cell distribution has a decisive influence on the validity of the experiment. The introduction of the cells into a fully assembled microfluidic system requires either some fingertip sensitivity or constructive mechanisms that ensure an even cell distribution. The following methods for cell trapping can be distinguished: hydrodynamic, optical, magnetic, electrical or acoustic.